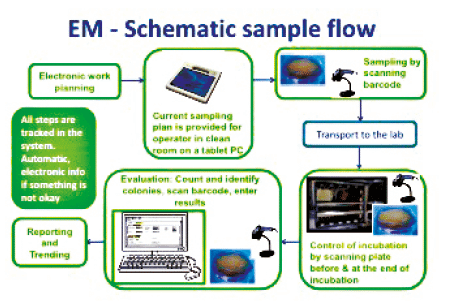
This would access the environmental conditions in particular the microbiological and particulate quality of the pharmaceutical clean room.
Cleanroom environmental monitoring program.
Webinars will be available for unlimited on demand viewing after live.
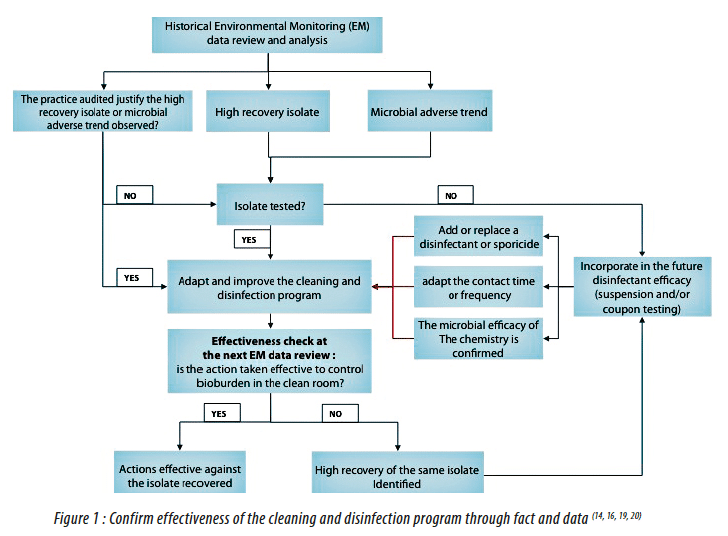
An effective environmental monitoring program alerts you when conditions contributing to excessive microbial levels rise due to ineffective cleaning sanitation or other personnel equipment issues.
Humans shed around 30 000 skin cells per hour 2 all of which are potential carriers of microbes.
Environmental monitoring em of cleanrooms is the microbiologist s responsibility and it requires making many decisions such as how often to monitor where to monitor what samples to take which culture media to use how long to incubate how to interpret data and which identifications to perform.
Cleanroom routine environmental monitoring.
Factors to consider range from regulations to identification of environmental isolates.
A robust system architecture from particle measuring systems pms ensures critical cleanroom environmental monitoring data are always available and protected when needed for release and reporting.
How beckman coulter can help routine environmental monitoring managers address the confusion.
Environmental monitoring is a tool that looks at the end results of the environmental control program.
The routine environmental monitoring program is a critical aspect of documenting the state ofcontrol ofthe facility recommendations for the selection ofsample sites to be used in the qualification program are provided.
Simple cleanroom environmental monitoring implementation with a modular and expandable design.
It requires a thoughtful scientific approach which assesses the risk of microbial contamination to the product.
List the basic content of a well designed effective and compliant em program.
Environmental monitoring programs are designed to estimate the microbial and particulate content of the room air and surfaces.
The difference between cleanroom classification and routine monitoring.
The differences between eu gmp and n america cgmp for aseptic manufacturing cleanroom contamination control.
Of course the fda mandates the air quality conditions for bio pharmaceutical production in cleanrooms.
In fact the real danger is the microbes on the human body.
Explain the basis of the four 4 phases of clean room transitional monitoring pre qualification through post qualification em and what each type stands for.
In this context the environmental monitoring data management course by eca academy 20 21 november in barcelona spain will present the basic methodology of evaluating the data using elementary statistical process control tools as well as the empirical approaches to set microbial control limits for cleanrooms.